During the last 16 months, following the data lock of October 5th. 2020, NWBO went into a quietness period (see article Quiet Period), where seemingly no kind of information revolving around the companys activities, have been published.
This has been continuously used as an argument by naysayers of NWBO’s 14 year long trial of DCVax-L, as the primary focus point, to induce fear and doubt into shareholders. Sadly, with success.
But while this has been continuously and successfully brought forward, has that postulate any kind of validity to it?
While the company has seemingly not been giving shareholders any kind of material information – which is also illegal according to regulations pertaining a quiet period – they HAVE nevertheless been informing shareholders “between the lines” in other ways.
So let’s have a further look into the timeline of events and announcements.
Annual Shareholders Meeting 2022 - Date ?
Last possible day for ASM according to the following: “no later than 15 months after holding the last preceding annual meeting, but no later than six months after the end of its preceding financial year".
June 4th. 2022
Marnix Bosch, PhD, Chief Technical Officer, Northwest Biosciences, will speak at ASCO (3:00 PM–4:00 PM) re: Dendritic Cell Vaccines for Cancer, and personalized dendritic cell cancer vaccines. -


May 10th. 2022
Linda M. Liau speaking at The New York Academy of Science’s Frontiers in Cancer Immunotherapy Conference re “Autologous Tumor Lysate-loaded Dendritic Cell Vaccination for Glioblastoma”
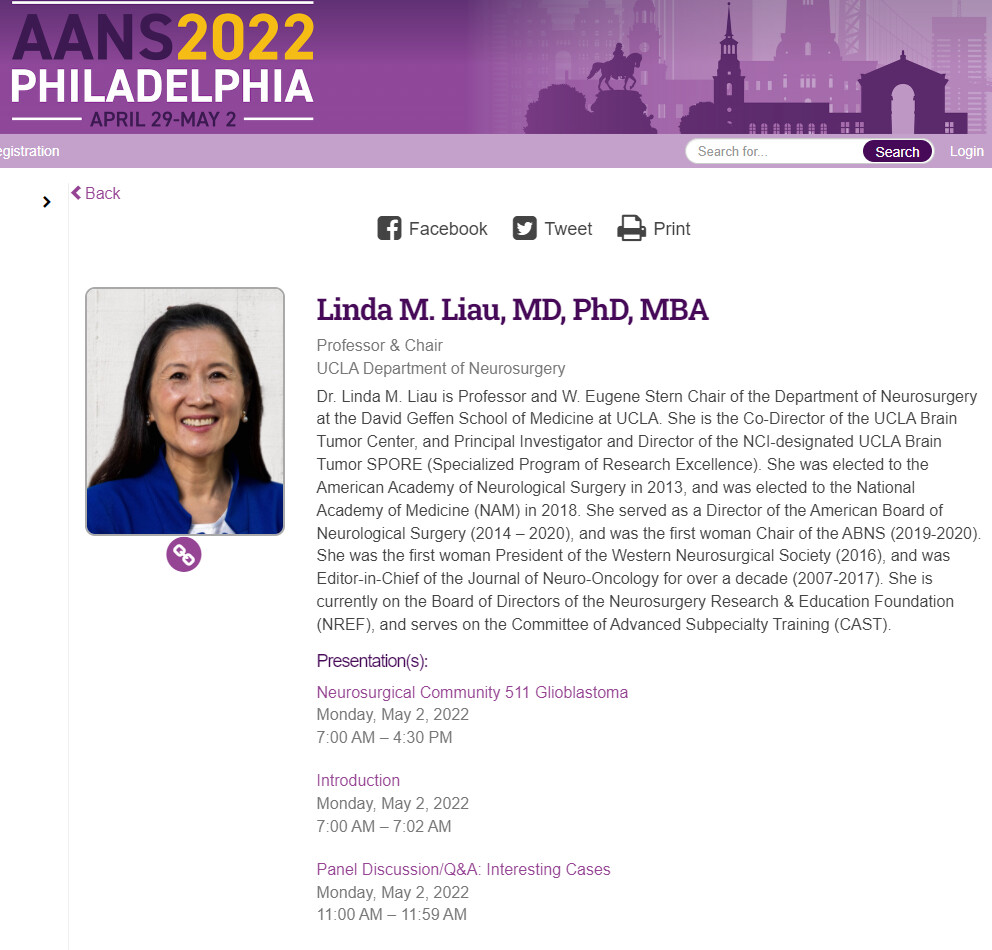
May 2nd. 2022
Linda M. Liau, distinguished UCLA professor, neurosurgeon, and brain tumor expert, who served as the principal investigator for Northwest Biotheraputics’ DCVax-L phase III study, speaking at AANS (American Association of Neurological Surgeons) re Glioblastoma.
April 20th. 2022
Patent update: “Issue Fee Payment” on April 19, 2022 (US16/841,197 - Dendritic Cell Generating Apparatus and Method). Issuance bill paid, just waiting for patent issuance.
March 31st. 2022
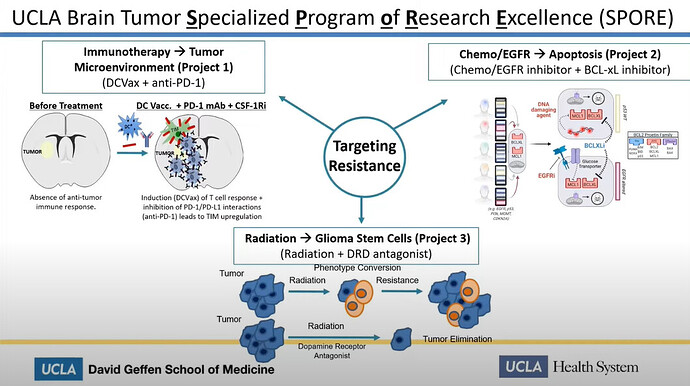
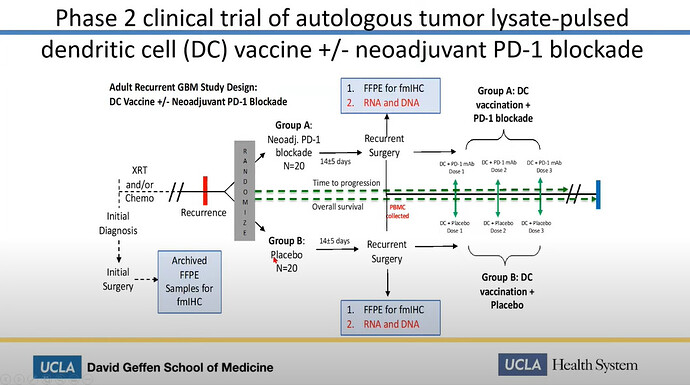
Linda Liau speaks about “Therapy induced resistance in glioblastoma” at University of Miami. She talks about the UCLA Spore 1 project, which directly mentions the use of DCVax-L in conjunction with PD-1 inhibitors.
In this presentation Linda Liau proves:
- The UCLA Spore 1 Project IS The combo trial with DCVax-L and Keytruda
- The trial is in Phase 2
- She is funded by Northwest Biotherapeutics
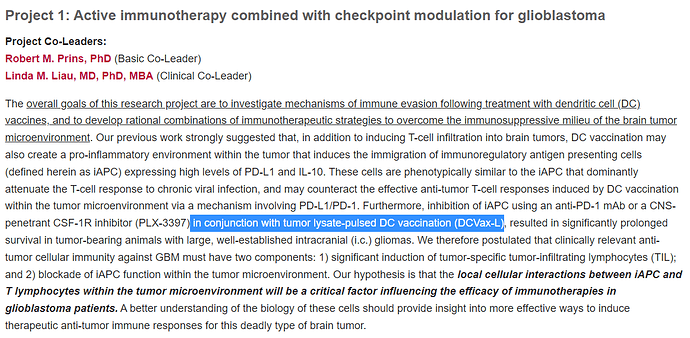
Actually the information here, mentions in “Aim 3” a Phase 2 clinical trial with Opdivo from Bristol Meyers Squibb as the PD-1 inhibitor (generic name Nivolumab).
But we know, that clinical trial was withdrawn before it started and was replaced with a clinical trial with Merck and their Keytruda PD-1 inhibitor instead (generic name Pembrolizumab), as shown in Linda Liaus slide below, where you can see Project 1 named together with the name DCVax + anti-PD-1)
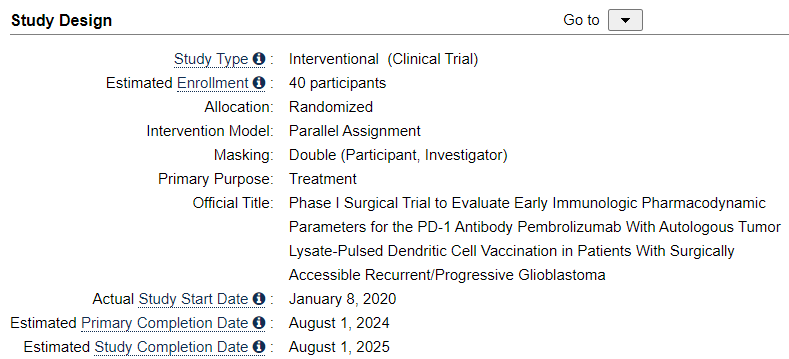
We can easily deduce, that the Spore 1 Project clinical trial is in fact the combo trial with DCVax-L. In a later slide, we see that the trial has 2 groups of 20 patients, 40 total.
Let’s look at the study of the combo trial of the ATL-DC and Pembrolizumab. It tells us indeed, there are 40 patients.
It also tells us, what is used in the combo trial. We know that Pembrolizumab is Keytruda. We also has confirmed a long time ago, that ATL-DC in these trials are DCVax-L. So we only need her presentation to show us, that the anti-PD-1 is Pembrolizumab.
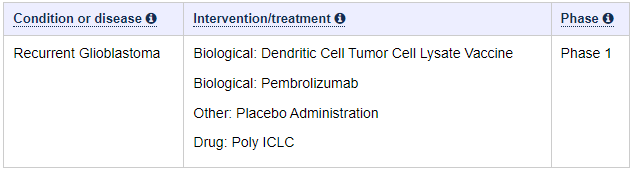
The answer to what that anti-PD-1 is, is shown at a later slide, with red ATL-DC + Pembro.
We even get “thrown a bone”, that the trial has gone from Phase 1 to Phase 2. Just look at the headline of the slide further above.
“Phase 2 clinical trial …”
And to even further prove Linda Liaus “allegiance” to Northwest Biotherapeutics we get her disclosures in the beginning of the trial.
NORTHWEST BIOTHERAPEUTICS
March 22nd.
February 9th. the article External Control Arms in Oncology: Current Use and Future Directions, was published in Annals of Oncology.
An article written by 12 authors ALL affiliated with U.S. Food and Drug Administration, also known as FDA. An article that can not be seen as anything but FDA green lighting the use of ECAs and at this point serves as nothing short of a “stamp of approval” for NWBO and their trial design.
That article has now been republished as an “Editors Choice” article.

March 22nd.
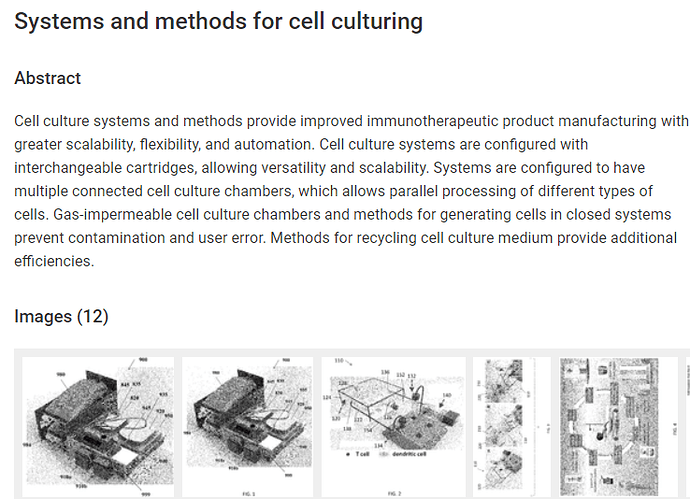
Two patents are getting “correlated”. A notice of allowance for application number 16/841197 " Dendritic cell generating apparatus and method" and application number 11268058 " Systems and methods for cell culturing". The patents are for EDEN and BATON.
Below an excerpt, but you ought to follow the discussion on Ihub for some mind blowing possible future developments, that could increase the value of NWBO considerably.
March 8th.
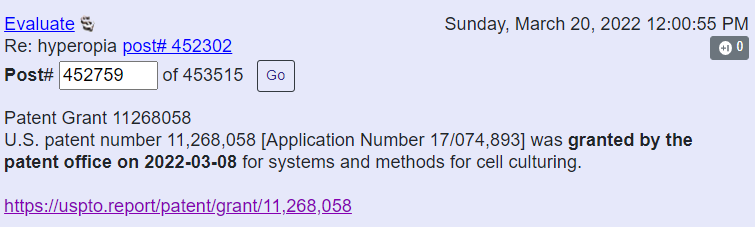
Flaskworks patent issue date on March 8, 2022: US17/074,893 - “Systems And Methods For Cell Culturing”.
March 5th.
Flaskworks patent application “Duty Cycle For Cell Culture Systems” published in New Zealand NZ784855 and National Phase Entry in Australia AU2020329966
March 2nd.
Dr. Marnix Bosch speaks at the Brain Tumour North West Retreat, a yearly cancer symposium, which is attended by british gbm clinicians. It seems he gets registered late for a 2 hours session, where he is to speak of “Dendritic Vaccine”.
The news is not in any way published.
It happens to correlate with a british article same day in the magazine Brain Tumour Research


which essentially says the same thing about the production in Sawston, as the article in the same magazine from February 26th. with the addition of Helen Bulbeck from Brainstrust, saying:
"Our hope is that this will become standard of care - so available on the NHS - but this will take time. We are always pleased to hear news of potential new therapeutics but to get them to benefit the majority of patients remains costly and needs the buy-in of a number of key stakeholders.”
March 1st.
FDA news release: FDA Clinical Trial Guidances Share Biden Administration’s Goals for Advancing Development of Cancer Treatments
March 1st.
Lancet article: Progression-free survival: it is time for a new name
February 26th.
Brain tumour research article: UK vaccine production 3D and dexamethasone
February 25th.
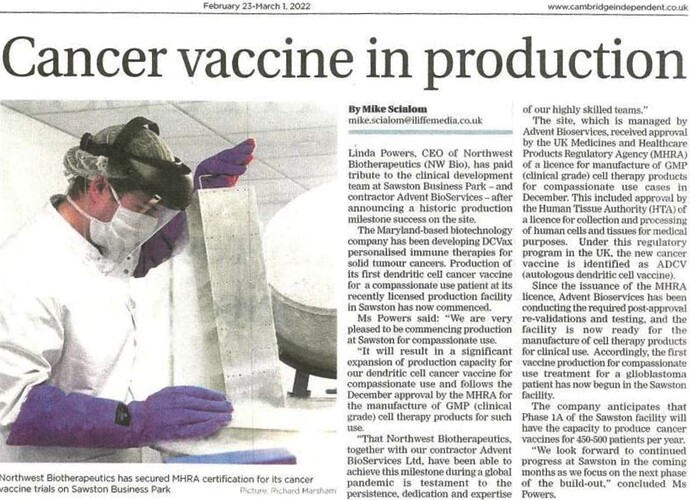
Cambridge Independent article: Sawston Business Park becomes world-class facility producing NW Bio cancer vaccine
February 24th.
Mexico patent attorney stated on Feb 18, 2022 (MX/a/2018/000056): “Third: Consider patent application MX/a/2018/000056 admissible, as there is no impediment. Fourth: When appropriate, grant the corresponding patent title.”
February 23rd.
Cambridge Independent news paper article.
February 18th.
Precision Vaccinations article: Brain Cancer Vaccine Candidate Production Launches in the U.K.
February 17th.
Article in Business Weekly
“A world first cancer vaccination has started production in Cambridge. US biotech pioneer Northwest Biotherapeutics (NW Bio) is steering the historic breakthrough by developing DCVax® personalised immune therapies for solid tumour cancers.”
February 17th.
NWBO PR
February 17th.
Stock Titan article
February 17th.
Flaskworks patent issue date on March 8, 2022: US17/074,893 - “Systems And Methods For Cell Culturing”.
February 15th.
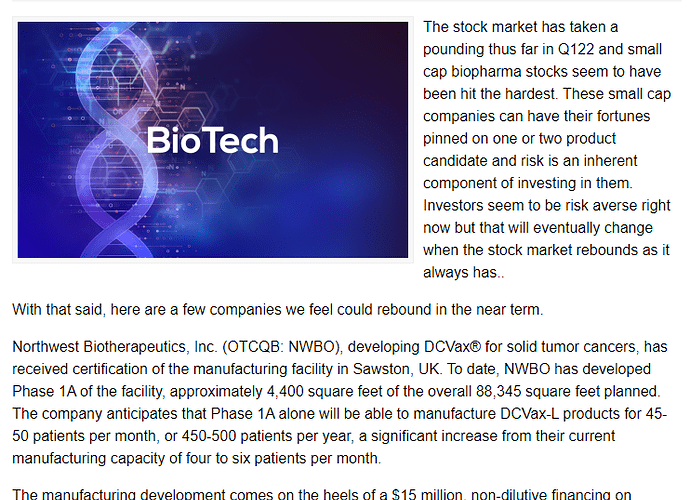
Traders News Source article: Biopharma Companies Setting up for a Rebound in the Near Term
February 9th.
Gov.uk article: Health and Social Care Secretary Savid Javid - World Cancer Day speech
"And that’s why I’ve published today a call for evidence that will inform a new 10 Year Cancer Plan for England."
February 9th.
Flaskworks patent application “Cell Culture Systems and Uses Thereof” published in 2022 by EPO and 3 other countries (Brazil, Republic of Korea, China).
February 6th.
Report Global Glioblastoma Multiforme Drug Forecast and Market Analysis to 2030 - ResearchAndMarkets.com
DCVax-L, regorafenib, and paxalisib are the three pipeline agents expected to generate the highest sales for GBM from 2020-2030.
February 1st.
Flaskworks patent application status with “Issue Fee Payment Received” - US17/074,893 - Systems And Methods For Cell Culturing
January 26th.
Interview in China Underground with Linda Liau
.
January 26th.
Article “Neoantigen Cancer Vaccines: Generation, Optimization, and
Therapeutic Targeting Strategies”
In a large phase III clinical trial for DCVax-L (Northwest Biotherapeutics), an autologous tumor lysate
dendritic cell vaccine in patients with newly diagnosed glioblastoma, the vaccine was
found to be safe and showed efficacy in extending overall survival of treated patients
January 26th. 2022
Larry Smith from Smith on Stocks, releases a report about “anything” NWBO.

January 26th. 2022
The Cambridge Independent has an article about the MHRA certification of Advent BioServices.
It starts out
The news that Northwett Biotherapeutics has secured MHRA certification for its manufactoring work in the Sawston Business Park is a triumph for Advent Bioservices, which has completed the laboratory facilities to UK regulatory standards on behalf of the US-based clinical stage biotechnology company…
January 24th. 2022
A Flaskworks patent application status with “Notice of Allowance”: : US17/074,893 - Dendritic cell generating apparatus and method
January 23rd. 2022



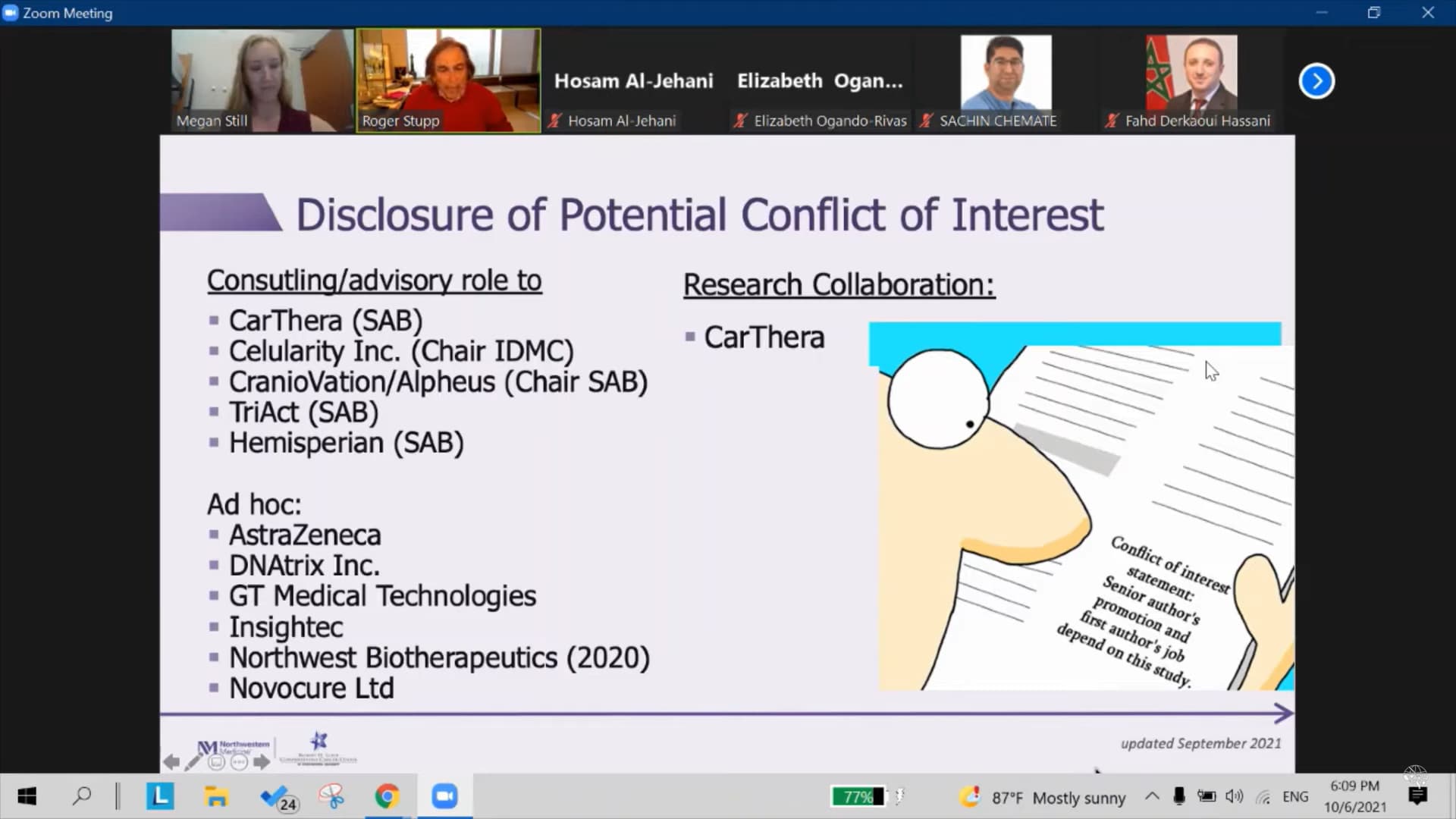
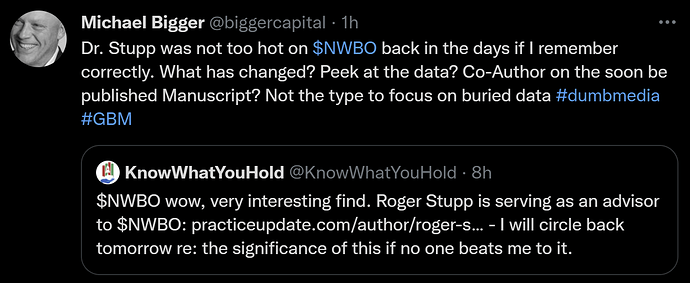
It is found out, that dr. Roger Stupp, the man behind The Stupp protocol, which became standard of care for the treatment of glioblastoma (GBM) since its publication in 2005, became an advisor to NWBO BEFORE September 26th. 2020..
The Way Back Machine crawls the internet and saves checkpoints in time for websites and web pages. We can see, that a checkpoint exists the 26th. September 2020, where the information regarding dr. Stupp is updated. The checkpoint before is all the way back to November 5th 2019. So we can only assert, that the disclosure happened in this time interval, not pinpoint the exact date.
It was in September 2020, that NWBO changed its strategy of going for “a quick” TLD, to instead “opt for a publication” in a medical magazine to support the release of TLD. Question is, if dr. Stupp had any saying or advice in that decision?
The revelation was found under “Disclosures” in PracticeUpdate
And yet again in a video (click image to play) with dr. Stupp from January 9th. this year, a zoom meeting that took place October 6th. 2021, one day after data lock. As you can see from the image, the disclosure mentioned was updated September 2021.
Another piece of important major news, found by Evaluate, 10baggerz and Lykiri on Ihub.
Investor Michael Bigger had the following remarks on his Twitter account:
January 11th. 2022
Patent granted by Canada. Canada application CA2632263 - Compositions And Methods For Inducing The Activation Of Immature Monocytic Dendritic Cells
January 11th. 2022
AdventBio announces their new Cryostore and Freezer facility at Sawston. Head of Production at AdventBio, Kelly Balsom, responds with "Going live!"
Lykiri from Ihub wrote London Neurosurgery Partnership about the availability of DCVax-L and they answered:
Yes it is available privately to everyone who is suitable for the treatment by Professor Ashkan in our group. There is not usually a delay with this once a consultation with Prof Ashkan has been booked and the treatment options discussed.
So Sawston HAS gone live and is capable of producing DCVax-L and treat more patients under compassionate use.
January 9th. 2022 IMPORTANT UPDATE:



New article in Annals of Oncology, External Control Arms in Oncology: Current Use and Future Directions
Author: P.S. Mishra-Kalyani and 11 co-authors, among these dr. Richard Pazdur, are ALL affiliated with U.S. Food and Drug Administration, also known as FDA. As one wrote on IHub:
Richard Pazdur, M.D. is the director of the FDA’s Oncology Center of Excellence (OCE), which leverages the combined skills of the FDA’s regulatory scientists and reviewers with expertise in drugs, biologics and devices to expedite the development of novel cancer products. In his role as director of the OCE, Pazdur is responsible for leading the effort to develop and execute an integrated regulatory approach to enhance the cross-center coordination of oncology product clinical review.
This FDA paper on External Control Arms (ECAs) used the 2019 draft guidelines on substantial evidence as a corroborative reference, as quoted:
Potential applications may include a study design for a clinical question where the natural history, morbidity, or mortality of the disease is well characterized, highly predictable, the expected effect size of the investigational treatment is high, and the outcome precisely measured [12]
A direct endnote to the draft guidance
U.S. Food and Drug Administration. FDA Guidance for Industry: Demonstrating Substantial Evidence of Effectiveness for Human Drug and Biological Products (December 2019)
This is as “good as it gets” of having FDA approving external controls, thus a big thumbs up from the FDA to NWBO and its trial design.
We don’t have to spell out, that this is huge.
Comment from ATLnsider on Ihub:
I believe that because of the crossover issue, and the fact that it was unethical (lack of clinical equipoise) to continue randomizing GBM patients to the control group, it was Dr. Pazdur and the FDA who suggested to Dr. Linda Liau and to NWBio that they use external control arms (ECAs) in the revised SAP for the DCVax-L Phase III trial, in addition to the randomized control group in the trial.
I also believe that the FDA was the first regulatory authority to give NWBio buy-in on the new SAP that revised the Primary endpoint to compare the DCVax-L trial treatment group OS, to the OS of ECAs. I believe NWBio was waiting for the UK, and Germany (EMA) to buy-in to the revised SAP and the use of ECAs.
NWBio announced data-lock on October 5, 2020 which indicated that NWBio had finally gotten buy-in to the use ECAs from all 4 regulatory authorities. Then on October 8, 2020 (3 days later) we discovered that the clinical trial registry in the EU (for the UK) had been updated to show the revised Primary and Secondary endpoints.
*Information courtesy of hbpainter, Flipper44, ATLnsider and more on Ihub. *
November 30th. 2021
Patent granted by Japan: JP2018523996 - Optimally activated dendritic cells that induce an improved or enhanced antitumor immune response
November 29th. 2021 - (2)
Announces $15 mill. Financing to help accelerate activities related to brain cancer program
November 19th. 2021
Patent granted by Japan : JP2018537949 - Activated Dendritic Cell composition for subjects with advanced cancer and methods for immunotherapeutic treatment
November 16th Q10 SEC filing.
Our operating costs also include the costs of preparations for new or expanded clinical trial programs, such as our planned Phase II clinical trials. The preparation costs include payments to regulatory consultants… Additional substantial costs relate to the maintenance and substantial expansion of manufacturing capacity, in both the U.S. and Europe
November 15th. 2021
A Flaskworks patent application status with “Notice of Allowance”: US17/074,893 - Systems And Methods For Cell Culturing
October 28th. 2021
Announces HTA License Issued and MHRA Inspection Conducted for Sawston, UK Facility
October 10th. 2021
During the year, AdventBio who is contractually hired by NWBO to do the work at NWBOs Sawston facility, hired or are in the process of hiring biobank manager, Facilities Coordinator, GMP production scientist, Process development Scientist, Quality Assurance Officer, Senior Production Scientist and cleaners.
September 30th. 2021
Patent granted by Israel: IL258071 - Methods Relating To Activated Dendritic Cell Compositions And Immunotherapeutic Treatments For Subjects With Advanced Cancers
September 17th 2021
Linda M. Liau, MD, PhD, MBA, Primary Investigator of the #DCVax-L #GBM P3 clinical trial (US) was inducted to the Asian Hall of Fame 2021
September 9th 2021
In an article from Siteman Cancer Center named “How is personalized brain tumor treatment different from more common protocols?” it is mentioned
Additionally, dendritic cell vaccines (including DCVax-L and DCVax-Direct) have shown success in treating cancer
August 8th.
The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. This new classification favors DCVax-L and thus also the endpoints NWBO settled for at data lock time.
The most important changes in WHO CNS5 involve the classification of gliomas, differentiating gliomas that occur primarily in adults from those that occur mainly in children.1-3 In adults, diffuse gliomas have been condensed into three types: 1) astrocytoma, IDH-mutant; 2) oligodendroglioma, IDH-mutant, and 1p/19q-codeleted; and 3) glioblastoma, IDH-wildtype. The change in the definition of glioblastomas will have important practical implications. Previously glioblastomas were diagnosed based on the histologic findings of microvascular proliferation and/or necrosis and included both IDH-mutated (10%) and IDH-wildtype (90%) tumors with very different biologies and prognoses. In WHO CNS5, glioblastomas will comprise only IDH-wildtype tumors.
August 3rd. 2021
Patent granted by Canada: CA2490245 - Tangential Flow Filtration Devices And Methods For Leukocyte Enrichment
June 16th. 2021
Patent granted by the Russian Federation:
RU0002749610 - Methods Related To Activated Dendritic Cells Compositions and To Immunotherapeutic Treatment of Individuals With Advanced Cancer
May 14th.
UK finalises biosimilar guidance designed to improve on EMA starting point
The UK’s Medicine and Healthcare products Regulatory Agency (MHRA) finalised its ‘Guidance on the licensing of biosimilar products‘, outlining the licensing requirements for biosimilars in the post-Brexit UK. The MHRA had previously foreshadowed in its draft guidance that comparative efficacy/safety trials would not be necessary for most biosimilars. The MHRA has maintained its position in this finalised guidance, stating that ‘Although each biosimilar development needs to be evaluated on a case by case basis, it is considered that, in most cases, a comparative efficacy trial may not be necessary if sound scientific rationale supports this approach
Comments regarding this
flipper44:
So the MHRA stance on biosimilars, no longer requires preclinical and/or clinical efficacy studies in most cases.Therefore, if NWBO/Flaskworks is trying to demonstrate biocompatibility with the manual method, logic follows it is a streamlined process.
May 12th. 2021 - (2)
Requesting MHRA certification of the Sawston facility to produce Good Manufacturing Practice (GMP) clinical grade medical products for patients.
May 1st. 2021 - (1)
Tamara Kondic gets attached from Donahoe Consultants as an uplisting consultant. She is an expert in uplisting companies to NASDAQ, where the other Donahoe consultant being attached – Bruce Poignant – is an expert on uplisting to NYSE.
How come NWBO attaches yet another uplisting expert, when already having one? Why not wait until being unblinded and knowing if the results of the trial is positive?
Well, we already know that of course, since NWBO got unblinded around March and the hiring of KP, almost certainly confirms the positive outcome.
NWBO are looking into possibilities of being uplisted, when top line data gets released. Both stock exchanges seems to be in play. Tamara Kondic and Bruce Poignant have also been working together on getting Core One Labs company listed on Nasdaq since February 2021.
April 15th. 2021
Patent publication date : US2020/054621
The present disclosure provides compositions and in vitro or ex vivo methods for obtaining an enhanced antigen specific Th1 immune response. The compositions can comprise activation enhanced dendritic cells or T cells produced in vitro. The methods comprise contacting immature dendritic cells with a maturation agent that comprises a dendritic cell maturation agent, interferon and an inflammation-activating lipid which can produce hyperactivated dendritic cells. The method can further comprise contacting the maturing dendritic cells with a predetermined antigen during maturation. An in vitro or ex vivo method is also provided wherein the hyperactive dendritic cells can be used to induce naive T cell activation, where the activated T cells can be formulated for administration to an individual in need of such treatment
Comments regarding this
ATLnsider:
It appears that an enhanced antigen specific Th1 immune response could be used to treat autoimmune diseases that are specific to the Th1 pathway.
Justin Keister, DABR Medical Physicist
It’s reasonable to hypothesize that DC activation could be involved in complex and not well understood immune responses.
Chiugray
Whereas CART-T unnaturally create super soldier T-cells assigned with a single task, DCVax is superior. It gives the General (dendritic cell) the information on who the insurgents are to attack. The benefit here is the General decides how best to fight and win a guerrilla war, knowing they have access to the full breadth of weapons available in the immune system, not just T-cells. There are considerations like collateral damage and ability to identify a constantly adapting enemy. The immune system does that. And not just during the fight, but the benefits of this continue into deploying systemic defense mechanisms afterwards.
April 1st. 2021 - (1)



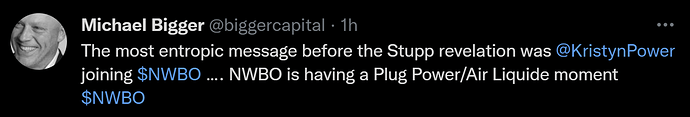
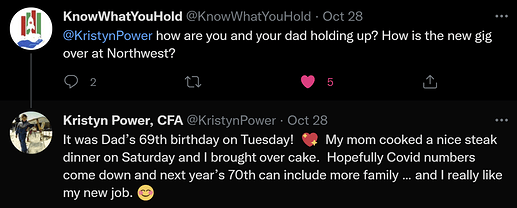
Kristyn Power gets hired by NWBO as a Program Director in Toronto. She left her manager job at Deloitte. KP has been an advocate for the DCVax-L vaccine, ever since her father was enrolled into the trial and is now to be considered a long time GBM survivor.
What is the big entropy “thingy” here?
Well, all logic tells us, that KP would not have agreed to the offer and left her old job, had she not in any way gotten some information, as to the success of the trial and thus being confident in having something relevant to do, when top line data gets revealed.
Therefore she was let in on the outcome by signing an NDA. In order for that to happen, the statisticians must have been ready doing the summaries of the six phases process, that NWBO have outlined on many occasions, that described the work to be done, from statisticians starts to analyze data, till TLD and publication are finally released.
Therefore, we can deduce, that NWBO must have been unblinded to the data before offering KP the job, that is, probably February/March.
With that follows, that we can also somewhat determine around when, NWBO was able to start the working on the scientific peer reviewed journal to be released simultaneously with top line data. If we use March 1st. as a starting point for that – for a moment not considering, that NWBO could of course have been working on a draft beforehand – that means NWBO has been working around 300 days on the publication.
If we look into the statistics on how long time it averagely takes, to get a publication with positive top line data done, from when data is available, we can look at this investigation in Jama Oncology regarding “Delays in the Publication of Important Clinical Trial Findings in Oncology”, which finding was (exact words):
Review of 100 pharmaceutical company press releases issued for clinical trial findings, the median delay from available results to publication of complete data was 300 days.
As you can see, this is not just a information entropy, in terms of being telling of not only the successful outcome of the DCVax-L trial, but also the explanation of the delay of releasing TLD.
The opposition of the bullish case of the DCVax-L trial, do not at all like this logic, since it of course not only tells us, the DCVax-L trial is a success, but also refutes the insinuation, that NWBO takes much more time writing the journal than seen in any other clinical trials. They don’t.
As a last comment to the hiring of KP, this event can be considered SO obviously “telling”, that one must also consider the possibility Linda Powers wanted to convey retailers the succesfull outcome of the trial during the quiet period, in a way that would never be questioned to be in violation of SEC rulings and regulations. To hire Kristyn Powers does exactly that.
March 16th. 2021 - (2)
Announces Development Completed for Initial Production Capacity of Sawston, UK Facility
October 8th. 2020 - (1).
Important discovery in the EU Clinical Trial register - Great Britain as well as Germany, that the DCVax-L Phase III clinical trial updated SAP, had revised the new primary and secondary endpoints.
The primary endpoint for the DCVax-L trial revised to Overall Survival (OS), not PFS , and the “second secondary” endpoint is PFS. Both primary and secondary endpoint to be evaluated in October 2020.
October 5th. 2020 - (2)
Data lock occurs.
Afterword.
We will continue to update the timeline above, as we find more events to document. Looking into how many patents NWBO seems to have in play - a portfolio around 265 globally - they seem very much aware of the importance of this. Having secured the IP surrounding the DCVax-L and DCVax-Direct vaccines is a huge sales parameter in case of NWBO ending in a buy out of positive top line data.