DCVax-L IS ATL-DC
Before we go any further, then there perhaps are even by readers, that asks the question …
What is DCvax-L?
For those, who are in need of a basic recap of what DCvax-L is, then get up to speed here.
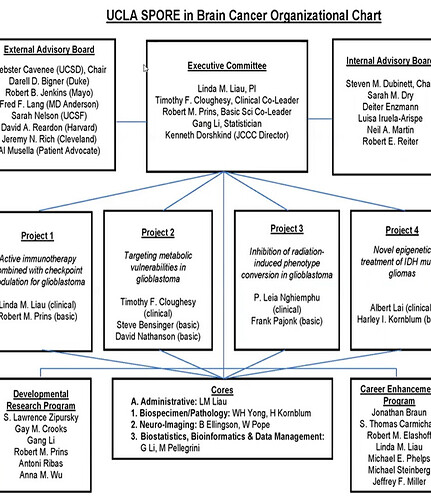
UCLAs SPORE program
On UCLA’s website you can read about the UCLA SPORE program.
The UCLA Brain Tumor Center:
The UCLA Brain Tumor Center is a designated Specialized Program of Research Excellence (SPORE) site funded by the National Cancer Institute. Dr. Linda Liau, who is Professor and Chair of the UCLA Department of Neurosurgery, Director of the UCLA Brain Tumor Center, and Principal Investigator of the UCLA SPORE in Brain Cancer, is helping to bring together basic researchers and clinicians to translate basic research from the laboratories into patient clinical trials much more quickly and effectively.
Objectives of SPORE
The objectives of the UCLA SPORE in Brain Cancer are to contribute significantly to progress in the diagnosis, prognosis, and treatment of brain cancer. These goals will be accomplished through diverse research projects involving mechanistic pre-clinical work and innovative clinical studies, with a particular focus on developing novel strategies to overcome the problem of treatment resistance. The SPORE program includes research for several brain cancer types, including low- and high-grade glioma, glioblastoma, and meningioma.
The 4 Projects
The broad, long-term objectives and aims of our brain cancer SPORE are as follows: 1) to investigate mechanisms by which brain tumors evade the immune system following immunotherapy, and develop rational combinations of immunotherapeutic strategies to overcome the immunosuppressive microenvironment of the brain tumor; 2) to elucidate the alterations in brain tumor metabolism that happens as a result of targeted therapy resistance, and exploit these metabolic vulnerabilities to induce intrinsic apoptosis of tumor cells; 3) to explore the concept of radiation-induced phenotype conversion of non-tumorigenic cells to glioblastoma-initiating cells as a mechanism for radiation resistance, and test new therapeutics to block this phenotype conversion; and 4) to investigate the pathways of resistance to IDH inhibitors and utilize novel epigenetic pathways to sensitize IDH-mutant gliomas to treatment. Please visit the “Research Projects” section of this website to learn more.
Who is the leader of project 1?

What is project 1?
Read more about it here
How do UCLA describe their immunotherapy research?
The UCLA Brain Tumor program](Biologics: UCLA Brain Tumor Center | UCLA Health) and the immunotherapy using DCvax-L and PD-1 inhibitors, is described like this:
We can see, that they explicitly mentions DCVax-L as the used vaccine, while they are not mentioning by name the PD-1 inhibitors used.
So let’s dive directly into a UCLA led clinical trial, a combo trial named

Which you can find on the website Clinicaltrials.gov. Where you can read further about privately and publicly funded clinical studies conducted around the world. Among these the DCVax-L and Keytruda combo trial.
As with all clinical trials, generic names are used, placeholders for the “real life ones”, we are concerning us self about, namely especially the dendritic vaccine DCVax-L.
In the following we are going to prove, that Pembrolizum is the PD-1 inhibitor Keytruda from Merck and that ATL-DC is the equivalent to DCvax-L.
Let’s get Pembrolizumab out of the way, with an image from Mercks own website.
That was that.
Next lets look at the desciption of the study from the ClinicalTrials site…
STUDY:
This phase I trial studies the side effects and how well of pembrolizumab and a vaccine therapy (ATL-DC vaccine) work in treating patients with glioblastoma that has come back (recurrent) and can be removed by surgery (surgically accessible). Immunotherapy with monoclonal antibodies, such as pembrolizumab, may help the body’s immune system attack the cancer, and may interfere with the ability of tumor cells to grow and spread. Vaccines, such as ATL-DC vaccine, may help the body build an effective immune response to kill tumor cells. Giving pembrolizumab and ATL-DC vaccine may work better in treating patients with glioblastoma compared to ATL-DC alone.
Facts: We got ourselves a combo trial, with ATL-DC and Keytruda and there are 40 patients enrolled
The FUD
It is important to look deeper beneath the hood of this, because there are forces in play, that seems very dedicated in trying to obscure the fact, that the name ATL-DC in this trial, is actually DCvax-L in its pure original unadulterated form.
There seems to be some kind of unity among the people with interests in stating and hoping that DCvax-L fails its phase 3 trial, that DCVax-L is NOT the vaccine used together with other PD-1 inhibitors, like Keytruda.
That’s why the fact is continously being contradicted, earlier by stating that ATL-DC in this combo trial was not at all DCvax-L, but another kind of vaccine. Then it was DCVax-L, but not the original “flavor”, but a somehow “other recipé”. People even tried to suggest, that Linda Liau had separated herself from the connection with NWBO and now worked on other combo trials, with this some kind of UCLA “enhanced by Linda Liau” DCVax-L vaccine, that NWBO somehow did not have the patent for.
It’s safe to say, that people have gone to extremes to disavow the 1-to-1 connection with ATL-DC in fact BEING DCvax-L in its wholly original state.
And no wonder.
If one has been watching the three presentations Linda Liau has done this year, all talking about the astonishing efficacy of DCVax-L in combinations with PD1-inhibitors (adjuvants) like Mercks Keytruda (the generic name Pembrolizumab), then this is definitely a key point in the whole fable of DCvax-L being the best to hit humanity since bread came sliced.
What is a PD1-inhibitor then?
Immune Checkpoint Inhibitors and Their Side Effects
An important part of the immune system is its ability to tell between normal cells in the body and those it sees as “foreign” (such as germs and cancer cells). This allows the immune system to attack the foreign cells while leaving normal cells alone.
Part of how the immune system does this is by using “checkpoint” proteins on immune cells. The checkpoints act like switches that need to be turned on (or off) to start an immune response. But cancer cells sometimes find ways to use these checkpoints to avoid being attacked by the immune system.
Medicines known as [monoclonal antibodies can be designed to target these checkpoint proteins. These drugs are called immune checkpoint inhibitors (or just checkpoint inhibitors).
Checkpoint inhibitors don’t kill cancer cells directly. They work by helping the immune system to better find and attack the cancer cells, wherever they are in the body.
Medicines that target different checkpoint proteins are now used to treat some types of cancer. All of these drugs are given as an infusion into a vein (IV).
PD-1 is a checkpoint protein on immune cells called T cells. It normally acts as a type of “off switch” that helps keep the T cells from attacking other cells in the body.
Monoclonal antibodies that target PD-1 can block this binding and boost the immune response against cancer cells.
PD-1 inhibitors
Examples of drugs that target PD-1 include:
- Pembrolizumab (Keytruda developed by Merck )
- Nivolumab (Opdivo developed by BMY Bristol Meyers Squibb)
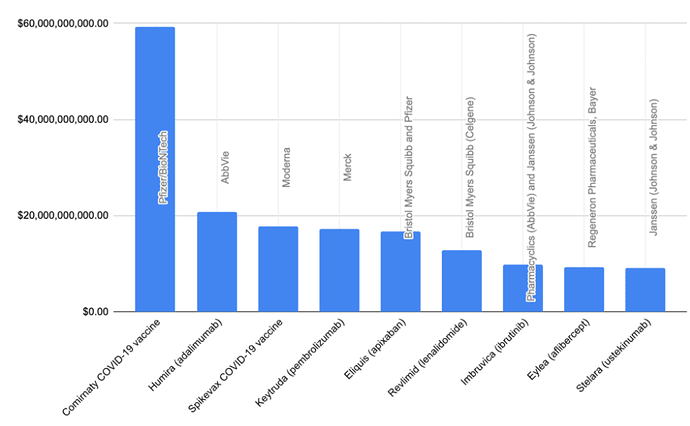
Keytruda has been the golden egg of Merck for quite some years now, and below you can see its revenue for 2021 as the 4th. biggest revenue maker of pharmaceuticals. You can also see, that its name is also Pembrolizumab. BMY’s Opdivo (Nivolumab) was number 12.
Keytrudas revenue was $17,186,000,000.00 and Opdivo was $7,523,000,000.00.
The DCVax-L and Keytruda combo trial
ATLnsider from IHub wrote about this:
"When we take a closer look at the UCLA - Merck Phase I clinical trial for recurrent Glioblastoma Multiforme (rGBM), with a treatment that combines an autologous tumor lysate-pulsed (ATL)-dendritic cell (DC) vaccine with Pembrolizumab (Keytruda), we can see the obvious fact that ATL-DC is the generic name for DCVax-L.
And we can see the same naming from the phase 3 clinical trial of DCVax-L, which we are currently awaiting TLD for.
ATLnsider commented further about the combo trial:
DCVax-L is the trademarked name for NWBio’s autologous tumor lysate (ATL) dendritic cell (DC) vaccine. ATL-DC is the generic name for DCVax-L. NWBio is not producing DCVax-L for this UCLA trial, through its commercial CMOs (Cognate & Advent Bioservices). This is a small Phase I clinical trial with only 40 patients, and there is only 1 clinical trial site (UCLA). There is no need for large-scale commercial production capacity for this trial. UCLA has the production capacity to make the vaccines on-site, in its own laboratory."
The trial is updated March 31st. 2022 and the status is Recruiting
These are the sponsors and collaborators.

You can see, that Merck is of course a collaborator, being the owner of Keytruda.
The Sponsor Jonsson Comprehensive Cancer Center, IS UCLA
![]()
As you can see in the study section above, it is only mentioning the names ATL-DC and Pembrolizumab. We’ve already established that Pembrolizumab is Keytruda.
ATLnsider wrote:
I personally believe that UCLA, Merck & NWBio want to purposely hide, obscure & downplay the connections between the 3 entities, and hide this DCVax-L + Keytruda combination clinical trial in plain sight. This allows NWBio to focus on releasing TLD, and prevent Merck & NWBio from having to answer questions about the interest they may have in each other.
In short, we shall walk you through why ATL-DC in this trial is the equivalent of DCVax-L.
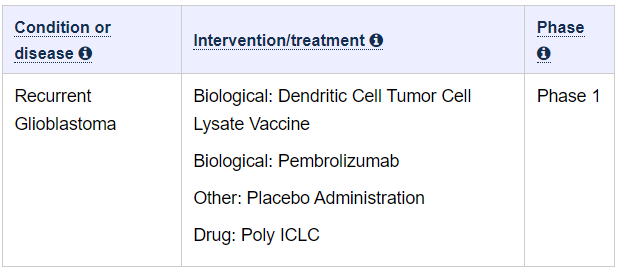
We can see that the study is in phase 1 and we will also show you, why in fact it has changed status to phase 2.
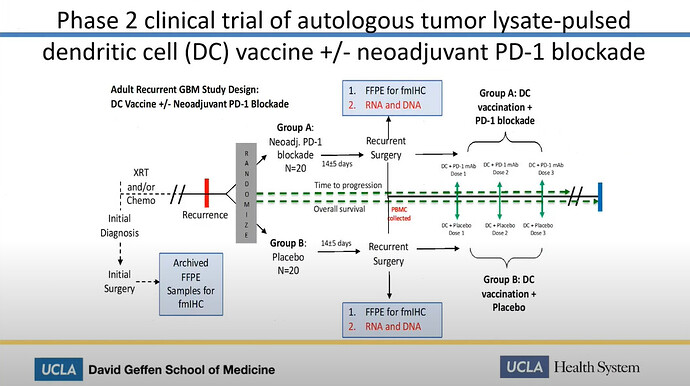
Study Design
Look at the number of patients. 40 has been enrolled. That figure will be important to remember in the following.
There are two control arms
Look at the generic naming used of DCVax-L

And look at the drug also being administered. It will be important in the following.
![]()
The evidence your honor
Exhibit A: Article UCLA Health
"As part of our National Cancer Institute-funded UCLA Brain Tumor SPORE (Specialized Program of Research Excellence), we recently opened a novel clinical trial to investigate the safety and efficacy of combining a personalized dendritic cell vaccine with an anti-PD1 immune checkpoint inhibitor, said Dr. Liau
Dr. Timothy Cloughesy says in this video clip at around 1:30
We have four different trials, one of them is an immune therapy project, that is run by Dr. Prins and Dr. Liau.
We have seen that UCLA has one project dedicated to testing ATL-DC in connection with a PD-1 inhibitor.
Exhibit B: Merck CEO Kenneth Frazier in article
For one, the company is looking ahead to coformulations, combinations and other routes of administration for Keytruda that could lengthen the PD-1 king’s patent life, he noted. And for two, the company has a pipeline full of candidates it thinks can help fill in the revenue gap once the drug does lose exclusivity.
Merck is looking for combinations together with its Keytruda PD-1 inhibitor.
Exhibit C: The Phase One Foundation Connection
Let’s take a look at one of the collaborators again, namely Phase One Foundation, and this article on their website.
Cancer researchers receive grant to develop immune-based therapies for deadly brain tumors.
Researchers from the UCLA Brain Tumor Center received a $400,000 grant from the PHASE ONE Foundation to support their research in developing immunotherapies for glioblastoma, an aggressive and fast-growing type of brain tumor.
The funding will help open a clinical trial testing a combination treatment strategy using checkpoint inhibitors in conjunction with a personalized dendritic cell vaccine, which was developed by Liau at UCLA. The team hopes by combining the two treatments they will be able to create a new way to treat people with brain cancer, as well as develop new ways to track the immune response.
“We already have had preliminary success using checkpoint inhibitors to treat patients in a previous clinical trial,” said Cloughesy, who is also a scientist at the Jonsson Cancer Center. “By combining the two immune-based treatments, we hope to bring in more T-cells that will attack cancer cells that would otherwise go unnoticed by the body’s immune system.”
By clicking on the link “was developed by Liau” above, you will be taken to this page on UCLA Health website.
On this page it says
The vaccine, known as DCVax-L …
Thereby effectively combining the statement
… a combination treatment strategy using checkpoint inhibitors in conjunction with a personalized dendritic cell vaccine** , which [was developed by Liau] at UCLA
With
The vaccine, known as DCVax-L
Exhibit D: Overview of Brain Tumors (video)

Take a look at this video by Dr. Cloughesy - working with Linda Liau on the SPORE project - at UCLA, that was released about 2 months ago, then go to the 1:30 mark to see that DCVax (ATL-DC) is the dendritic cell vaccine that UCLA uses in combination with a PD-1 inhibitor (Pembrolizumab / Keytruda).
Then go to the 27:35 minute mark to listen and see that Dr. Cloughesy also believe that DCVax can not only be used to treat GBM tumors, but it can be used to treat all solid tumor cancers.
Then go to the 31:10 mark to see what vaccines are used by UCLA to get the immune cell to the tumor to fight the tumor. The only dendritic cell listed is DCVax. Also, the peptide vaccines listed (ICT & CDX-110), have already failed their clinical trials for ndGBM.
Exhibit E: Matt Henkel
Matt Henkel who was a gbm patient, confirmed that DCVax-L was being used in a double blind study, himself receiving his third dose of DCVax-L.
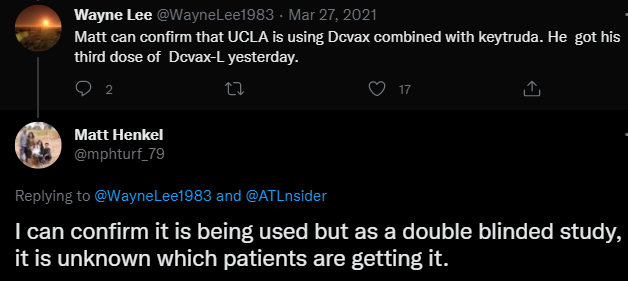
And he retweeted ATLnsiders words

Exhibit F: UCLA Brain Tumor Conference " Understanding Clinical Trials"
Listen to the video here around 43:13
Quote:
We have a trial that is using the dendritic cell vaccine plus or minus a drug called pembrolizumab. So the patients will all receive the DCvax, but they may or may not receive the pembrolizumab.They may get pembrolizumab or the placebo. That is a trial we have. Thats a trial that does go on, that uses a placebo. But it also uses a treatment that is the dendritic vaccine.
Exhibit G: UCLA Brain Tumor Virtual Conference (part 2) Mar 12, 2021
Lykiri from Ihub had this posting about questions during the Q and A session.
Questions
"I have a few questions about this trial at UCLA: Pembrolizumab and a Vaccine (ATL-DC) for the Treatment of Surgically Accessible Recurrent Glioblastoma.
This trial with Merck’s Keytruda coupled with DC VAX L has been ongoing for about 14 months.
How many patients are enrolled to date?Is there radiation or chemotherapy (temozolomide or avastin to help reduce swelling and prevent tumor growth)?
What has UCLA learned from this trial to date?
Can someone from the UK participate in this trial?
Why is the name of the vaccine “ATL-DC vaccine” and not DCVax-L? Are both generic the same?"
Answer from Nghiemphu, Phioanh [Leia] M.D.
As long as a patient can come to UCLA to have surgery for tumor resection and come to UCLA every 6 weeks for pembrolizumab or placebo infusion, they can be enrolled into this trial. It is ongoing and we are still enrolling patients. We do not have results as it is still too early.
The vaccine names are different but it is the same vaccine.
Exhibits from Linda Liaus Presentations from 2022
Exhibit H: Disclosures from Linda Liau of funding
From presentation slide in February 2022. Grant/funding from Merck
From presentation slide in March 2022. Grant/funding from Northwest Biotherapeutics
Exhibit I: The combo trial is in phase 2
The slide shows and Linda Liau says, that the combo trial is in Phase 2
With that we went on to do a clinical trial and this is the clinical trial of paradigm, it’s basically an ongoing trial we have right now. It’s a phase 2 clinical trial of autologous tumor pulsed dendritic cells plus or minus neoadjuvant PD-1 blockade.
Exhibit J: Liau IS talking about the Combo trial DCVax-L and Keytruda
Look at the excerpt here from the slide above. Two groups. Each 20 patients
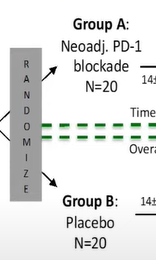
Look at the study information from the trial on the ClinicalTrials website
Thats right. 40 Participants
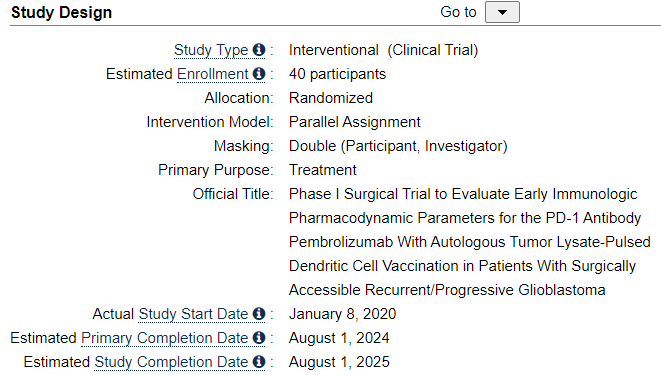
Exhibit K: Trial is active and recruiting
The trial was updated March 31st. 2022. It’s in recruiting status.
Correlate with National Cancer Institute website for the trial, which says trial is ACTIVE.

Conclusion
Beyond all doubt, the clinical combo trial with ATL-DC and Pembrolizumab, has the brand names DCvax-L and Keytruda. While websites has not updated phase from 1 to 2, Linda Liau states it in both slide and in talk, that the combo trial IS in phase 2. Status active and recruiting.
Next article
Due to the size of this article, we are going to look into the patents and rights of DCVax-L in our upcoming one.